Research
We study how immune regulation depends on molecular interactions of leukocyte surface receptors. The overall effect of a receptor can vary from activation to inhibition. It is critical to understand how this is controlled to interpret the role of a receptor in a complex biological system and for designing therapeutic intervention in autoimmune diseases and cancer. By studying selected receptors, we aim to identify both the specific niche the receptors occupy and the general paradigms.
We have developed methods for detecting low affinity interactions at cell surfaces. This led to the identification of the CD200/CD200R, CD47/SIRPalpha and CD48/2B4 interactions. All these interactions are being studied as therapeutic targets. One leukocyte surface receptor may interact with more than one extracellular or intracellular ligand. Thus it is important to establish a hierarchy as to which interaction occurs in a given setting. We have quantified interactions using surface plasmon resonance, which then allows us to relate the role of the interaction to functional outcomes in cellular experiments.
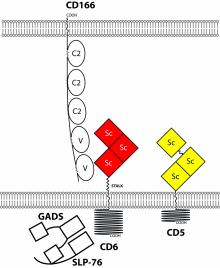
We currently focus on two receptors, CD5 and CD6 which belong to a family of leukocyte surface receptors which contain scavenger receptor cysteine rich domains. These receptors have both activating and inhbibitory effects on lymphocyte activation. We aim to understand the molecular basis of these dual effects and how they are regulated in human T cells.